Overview
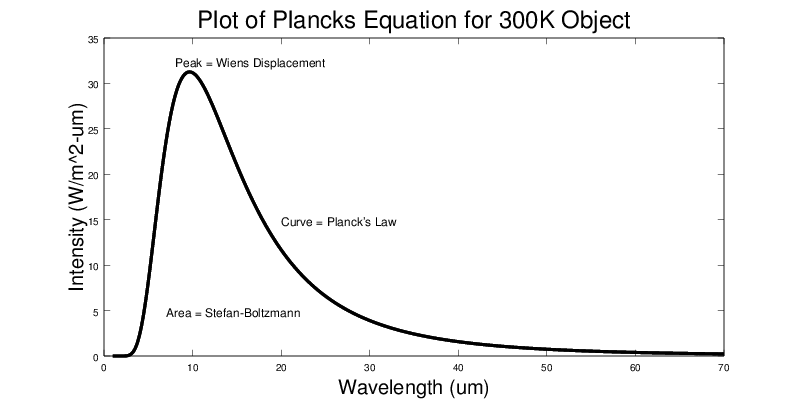
The Stefan-Boltzmann Law states that the total amount of energy radiated from a substance is a function of the amount of area exposed, the emissivity of that substance, and its absolute temperature. |
P = σ A e T4 |
P = the power radiated, in W |
σ = Stefan-Boltzmann constant = 5.670373 x 10-8 W/(m2K4) |
A = area of substance exposed, in m2. |
e = emissivity of substance, which is a number between 0 and 1 (no units). |
T = absolute temperature of the substance, in kelvin (K). |
Thermography deals with the use of thermographic imagers to study the thermal radiation from objects. In general, thermography covers the emission, detection, processing and display of thermograms, which are better known as thermal images. Thermal radiation is the electromagnetic radiation due to all objects having a temperature above absolute zero.
According to the abstract for the paper Metric Characteristics of Thermography, "Thermography is concerned with the pictorial representation of infrared radiation emanating from an object. The instrument that produces visible images of this thermal radiation is called a thermograph, and the permanent recordings of these images are called thermograms. The thermograph may thus be characterized as being an electro-optical imaging device that operates as a wavelength converter, translating spatial and thermal information about an object to the visible spectrum from that of the infrared. In addition, special processing techniques may be employed to enhance or identify certain signal characteristics as an aid for the user in evaluating the displayed data."
There are two ways in which thermography can be used. These are qualitative and quantitative. Qualitative refers to analyze an image without attempting to make quantitative measurements. For example, you might use a thermal image to look at your walls of your house to see if the insulation is properly installed. Quantitative means analyzing in order to make measurements, typically temperature measurements.
Difference between "heat" and "temperature"
Temperature refers to the degree of motion of the particles in a material, meaning the speed with which the particles move. Heat refers to the amount of energy stored in a body as motion among its particles and depends on density as well as temperature.1
How Heat Flows
Heat flows from hotter (more energy) to colder (less energy), and can flow in one, some or all of the following ways:
- Conduction
- Convection
- Radiation
How Do We Measure Temperature?
We can only measure temperature indirectly. This means that we use the heat energy to create some effect on something else. For example, we use the heat to change the resistance of a substance; this is the basis of the thermistor. For a particular substance, the greater the amount of heat energy contained within, the higher its temperature. There are four ways in which to measure (again, indirectly) the temperature of an object:
- Thermistor: Measures the change in resistance due to heat change.
- Thermometer: Measures changes in the volume of a substance due to heat change.
- Thermocouple: Measures the voltage change within a substance due to heat change.
- Thermal imager: Measures the thermal radiation from a substance.
Definitions
- Blackbody radiation: A theoretical object in which all radiation is absorbed and emitted.
- Thermal radiation: The electromagnetic radiation from an object due to its temperature being above absolute zero. There is some confusion as to whether the term "blackbody radiation" is synonymous with "thermal radiation". For example, one NASA website states that thermal radiation is the same as blackbody radiation. Most websites, including NASA, also state that blackbody radiation refers to a theoretical object that absorbs and emits 100% of the electromagnetic energy impinging it. This means that it has no reflectance and no transmittance.
- Thermogram: Image of the thermal radiation from an object.
Difference between "Infrared" and "Thermal"
Many people confuse "infrared" imaging with "thermal" imaging. However, the two are not always the same. While it's true that much of the thermal radiation from terrestrial objects is emitted in the infrared range, this is due to its temperature being in the vicinity of 300 K absolute temperature. It's not because all thermal radiation occurs in the infrared range. Again, thermal radiation refers to all electromagnetic energy emitted from an object due to its temperature being above absolute zero. Infrared refers to a range of the electromagnetic spectrum that covers from just above the radio wave range (roughly 1 mm wavelength, or 300 GHz in frequency), and just below the visible light range (roughly 0.78 microns).
Note that it's not redundant to refer to an "infrared thermal imager". This simply means that this type of imager is designed to capture, process and display thermal radiation emitted in the infrared range.
Footnotes
1. How Matter Emits Light - Blackbody Radiation(↑)
Emissivity
Thermography is the study of objects based upon their thermal radiation emissions. Again, thermal imagers measure thermal radiation, not temperature. The amount of thermal radiation that a thermal imager will see coming from an object is based on several factors, including:
- The temperature of the object.
- The emissivity of the object's surface.
- The distance between the object and the imager.
- The angle of the surface emitting the radiation with respect to the direction towards the imager.
- The amount of energy being reflected from the objects surface from the other objects with thermal radiation in the area, sometimes called the background radiation or Trefl.
Understand that the amount of energy coming from a particular surface is only due to the substances temperature and emissivity. Why, then, do we have all of these other factors affecting our thermal image? That's because we're collecting the radiation of the emission. That, in turn, is affected by factors such as distance, angle between the surface and our imager, and humidity (essentially water molecules in the air). There's also the fact that when imaging something, the object isn't the only thing emitting thermal radiation. Therefore, the amount of energy being reflected by the object under inspection is also a factor in the amount of radiation being seen by your imager.
Not that Mousy Kind of RAT...
The law of conservation of energy says that the energy impinging on an object will either be reflected, absorbed or transmitted (pass through). This creates the acronym RAT (reflected, absorbed, transmitted). The ratio of all three must equal one; otherwise energy is either being created or destroyed (violation of conservation of energy).
R + A + T = 1
In thermal imaging, many of the objects viewed are opaque. This means that the transmitted part is zero. Thus, all of the energy is either reflected or absorbed.
R + A = 1
If the object is not in equilibrium with it surroundings, meaning it is hotter or colder than its surroundings, then R + A != 1. If the object is hotter, then colder than its surroundings, meaning it is not in equilibrium, the energy absorbed by the object will cause the internal temperature of the object to increase.
This is where a theoretical object called a blackbody radiator comes in handy. Such a radiator perfectly absorbs every bit of energy impinging on it. This means that A = 1. Since R + A = 1, and since A = 1 for a blackbody radiator, this means that R (reflectance) = 0 for a blackbody radiator. It also means that, if the object is in thermal equilibrium with it surroundings, then all of the energy impinging on it must be absorbed then immediately re-radiated. If the energy is absorbed but not re-radiated, then the object's total energy will increase. This, in turn, means an increase in temperature, which means it is not in equilibrium.
The absorptance of an object has another word. That word is emissivity (which typically uses as a variable the Greek letter epsilon, ε). Emissivity is a ratio of the amount of energy an object absorbs and re-radiates versus the amount of energy a perfect emitter (meaning a blackbody radiator) would radiate. Differences in emissivity is the reason that two objects of the same temperature appear differently in thermal imagers. Emissivity is a ratio, as stated before, which means that it is numerically expressed as a number between 0 and 1. An object with 0 emissivity is one that does not absorb any radiated energy; this means that it is a perfect reflector. A value of 1 means that it absorbs and re-radiates 100% of the energy hitting it.
The amount of power radiated from an object is dependent upon its area exposed, its emissitivity, and its temperature. Therefore, given two substances which are at the exact same temperature and with the same amount of surface area exposed, the one with the higher emissivity value will appear warmer simply because it is giving up its heat energy more readily than the other substance. It will also cool off faster (assuming it is not generating energy internally) due to the fact that it will more readily radiate its energy away.
Also, the amount of energy radiated is a strong function of its temperature. As a matter of fact, the amount of energy radiated is a factor of the fourth power of its absolute temperature. Therefore, given even a slight increase in temperature, the amount of energy radiated will increase dramatically.
Finally, another key point about emissivity concerns a special case in which none of the energy incident on the object is transmitted through that object. Specifically, in this case, the emissivity is the reciprocal of the reflectance of an object. The higher the emissivity, the lower the reflectance and vice-versa.
Material Emissivity
The following table shows the average emissivity values for several substances.
Material |
Emissivity |
*
* *
* *
|
Material |
Emissivity |
Aluminum alloy, oxidized |
0.40 |
|
|
Brick, red rough |
0.93 |
|
|
Brick, fire |
0.75-0.80 |
|
|
Clay tiles |
0.33 |
|
|
Concrete |
0.94 |
|
|
Copper, oxidized |
0.87 |
|
|
Copper, polished |
0.07 |
|
|
Iron, oxidized |
0.74 |
|
|
Iron, unoxidized |
0.05 |
|
|
Paint, Aluminum |
0.27-0.67 |
|
|
Paint, Oil
|
0.92-0.96 |
|
|
Sandstone |
0.67 |
|
|
Tape, Electrical |
0.96 |
|
|
Wood |
0.80-0.90 |
|
|

